Table of Contents
Introduction
Environmental pollution is a simultaneous emerging problem as the modern civilization develops quickly. The problem is getting worse day by day.
There has also been an increase in the concentration of non-biodegradable pollutants and toxins. The threat posed by hydrocarbon contamination resulting from the activities of petroleum refineries, accidental releases of petroleum products, and natural phenomena is alarming.
In order to mitigate the risk of pollution hazards, effective management strategies have been undertaken. However, these strategies are not eco-friendly, are expensive, and are less effective.
On the flip side, by utilizing different microorganisms to clean contaminants from the environment and water, bioremediation is considered to be one of the most promising, popular, effective, and sustainable strategies in the field today.
In bioremediation, specific microbes feed and digest the toxic chemical pollutants. It is proving to be one of the most successful and environmentally friendly tools for cleaning locations inaccessible by humans.
An integral part of bioremediation is biotechnology, which provides natural mechanisms for decontaminating the environment, soil, and water. When contaminants are industrial wastes, biotechnology mechanisms are applied to bioremediation. Scientists are putting more effort into enhancing microbes’ ability to metabolize toxins.
Natural heavy metals in the environment
The term “Heavy metal” is applied to 53 of the 90 naturally occurring elements. Out of these, there are four essential micronutrients for plant growth: Copper (Cu), Manganese (Mn), Iron (Fe), and Zinc (Zn).
Essential heavy metals (Ni, Pb, Fe, Mn, Cu and Zn) are the micronutrients that are essential for important biochemical and physiological activities of plant growth
However, if their concentrations are higher than those required for normal growth, they will be toxic. Additionally, metals like Mercury (Hg), Cadmium (Cd), and Lead (Pb) are toxic in even very low concentrations.
Non-essential heavy metals (Cd, Hg, Pb, Cr and as) have no known biological or physiological function and are thus considered non -significant for plant growth and development
Heavy metal toxicity to humans and animals
Heavy metals in both categories are toxic to plants, humans, and animals in excess concentrations. Therefore, higher concentrations of both essential and non-essential heavy metals in the biological system could negatively impact growth and development as well as cause adverse health effects.
These metals may either exist as individual entities or in amalgamation with different soil constituents which could comprise of soluble metal ions in the solution or exchangeable ions that are absorbed onto the surface of organic or inorganic metal compounds like phosphates and carbonates
There are different effects associated with heavy metal toxicity, such as Selenium toxicity (9 mg/day), which can result in deformed fingernails in humans and alkali diseases in animals.
The toxic effects of Cadmium (200 micro-grams/kg-1 fresh weight) disrupt the normal functions of Zinc and Calcium. Cadmium toxicity is the cause of “itai-itai“, a multi-system disorder disease, which causes severe osteoporosis and bone fragility.
The “Minamata” disease (Japan 1953-60) can also be caused by mercury toxicity (>0.1 micro-gram/kg BW). Many people died because of mercury contaminated fish.
Tissue abnormalities were observed in the central nervous system, fetus and red blood cells as they are more susceptible to methyl mercury contamination (27-102 ppm).
A toxic dose of arsenic (3mg/day) can cause skin cancer, hyperkeratosis, hyper-pigmentation, black foot, and internal organ cancer.
Free radicals are produced by redox-active transition metals (e.g., Fe2+ and Cu2+). Essentially, they replace other essential metals in enzymes and pigments.
A few metal ions (Hg2+, Cu2+) can interfere with protein structure and function by reacting with thiol groups.
There are some metals that exist as radioactive isotopes (238U, 137Cs, etc.) that pose health risks.
Heavy metals concentrations in soils and their standard regulatory limits are shown in the table below:
| Elements | Concentration (mg/kg) | Standard Regulatory limit (mg/kg) |
|---|---|---|
| Arsenic | 0.1-102 | 20 |
| Cadmium | 0.1-345 | 100 |
| Chromium | 0.005-3950 | 100 |
| Copper | 0.03-1550 | 600 |
| Lead | 1-6900 | 600 |
| Mercury | 0.001-1800 | 270 |
| Zinc | 0.15-5000 | 1500 |
| Salt et al.,1998 |
Traditional methods of Heavy metal Amelioration
As for heavy metal remediation, the most common and conventional approaches are land filling, thermal treatment, excavation and burial, ion exchange, land farming, chemical extraction, soil washing, and groundwater extraction and treatment.
Traditional solutions are undesirable because they involve high costs, are intrusive in nature, can’t always be implemented, can destroy soil structure and CEC, destabilize ecosystems, are aesthetically unappealing, and produce mediocre results.
Phytoremediation of heavy metals
The concept of utilizing nature to clean nature is based on an environmentally friendly, cost-effective, solar-energy driven technology that is aesthetically pleasing and cost-effective.
What is phytoremediation ?
A method for removing, degrading, or containing chemical pollution from the soil, sediments, groundwater, surface water, and even the air using plants and their associated microorganisms.
Decontaminating polluted soil and water naturally through –
Use of green plants to remove pollutants from the environment or render them harmless
what is used in phytoremediation ?
Furthermore, soil microorganisms in conjunction with plants are also used for the remediation of organic and inorganic contaminants.
Phytoremediation has the following objectives:
- In order to reduce contamination levels of several pollutants in soil and water.
- For accumulating contaminants in harvested plants that can be removed and to improve or maintain the physical conditions of the site.
- Chemical and biological improvement of the soil.
Types of phytoremediation
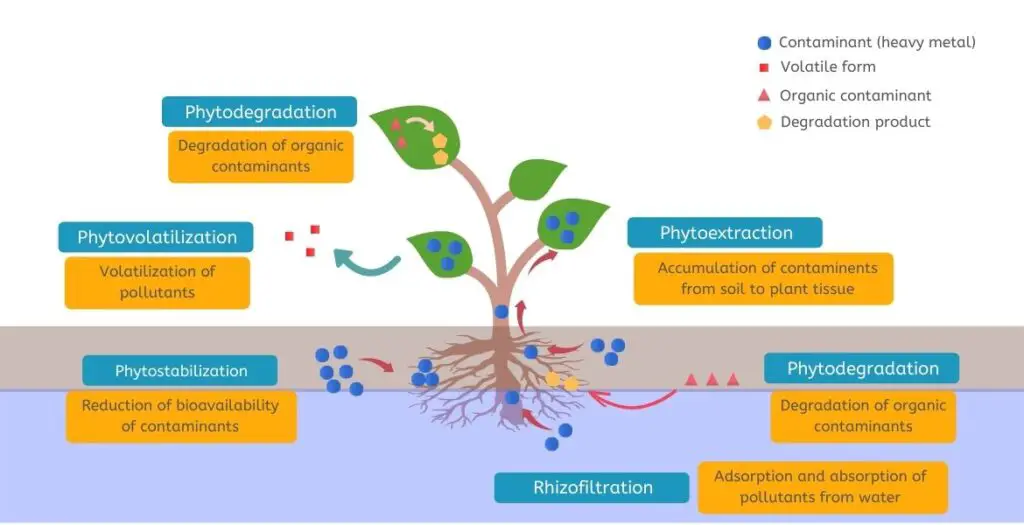
In the case of organic pollutants, phytoremediation uses mechanisms such as phytostabilization, phytovolatilization, rhizodegradation, and rhizofiltration.
For inorganic pollutants, the phytoremediation mechanisms are phytovolatilization, phytostabilization, rhizofiltration, and phytoaccumulation.
Following are some Phytoremediation types :
1 . Phytostabilization
Plant roots have the potential to stabilize, immobilize, and bind to the toxic particles in the soil, thereby reducing their bioavailability.
Plant species can immobilize contaminants in the groundwater and soil by absorbing and accumulating pollutants, or precipitating metal residues within the root zone.
Ideally, this process is used to remove metals toxicants from soils, sediments, and sludge.
2 . Phytoextraction
Through the process of phytoextraction, certain plant species are able to accumulate high concentrations of metal pollutants, along with surplus nutrients in harvested shoots and roots, from the substrate or the contaminated soil.
This process is optimal for metals, non-metals, radionuclides, metalloids, organics pollutants in soils, sediments, and sludge medium
3 . Phytovolatilization
The ability of plants to absorb and subsequently volatilize polluting particles into the atmosphere. Metal particles in soil, groundwater, sediments, and sludge can be removed using this process.
4 . Phytotransformation
It involves the metabolic uptake of pollutants along with nutrients by the plant and the subsequent breakdown of contaminants either internally by plant metabolism or externally by effects induced by the plant
In soils, groundwater, sediments and sludge, this process can be utilized to reduce complex organic molecules, by breaking them down into simpler contaminant molecules.
5 . Rhizofilteration
Rhizofiltration is the process of taking in toxic substances, along with excess nutrients, from the growth medium or contaminated site by the roots of plants.
Polluting particles are either deposited upon the roots of the plant or are absorbed into the root zone by precipitation or adsorption.
The process is ideal for removing excess nutrients, metals, and radionuclide pollutants from surface water, ground water, and wastewater.
6. Rhizodegradation :
Rhizodegradation is the process by which soil contaminating particles are broken down through the action of microbes as well as additional absorption of pollutants by the roots of the plant.
It utilizes microorganisms to consume and metabolize organic substances and provide nutrition and energy.
The natural substances released by the plant roots such as acids, sugars and alcohols contain organic carbon moiety that serves as energy source for resident soil microorganisms and create a thick root mass that takes up high quantities of water
Mechanism of Phytoremediation
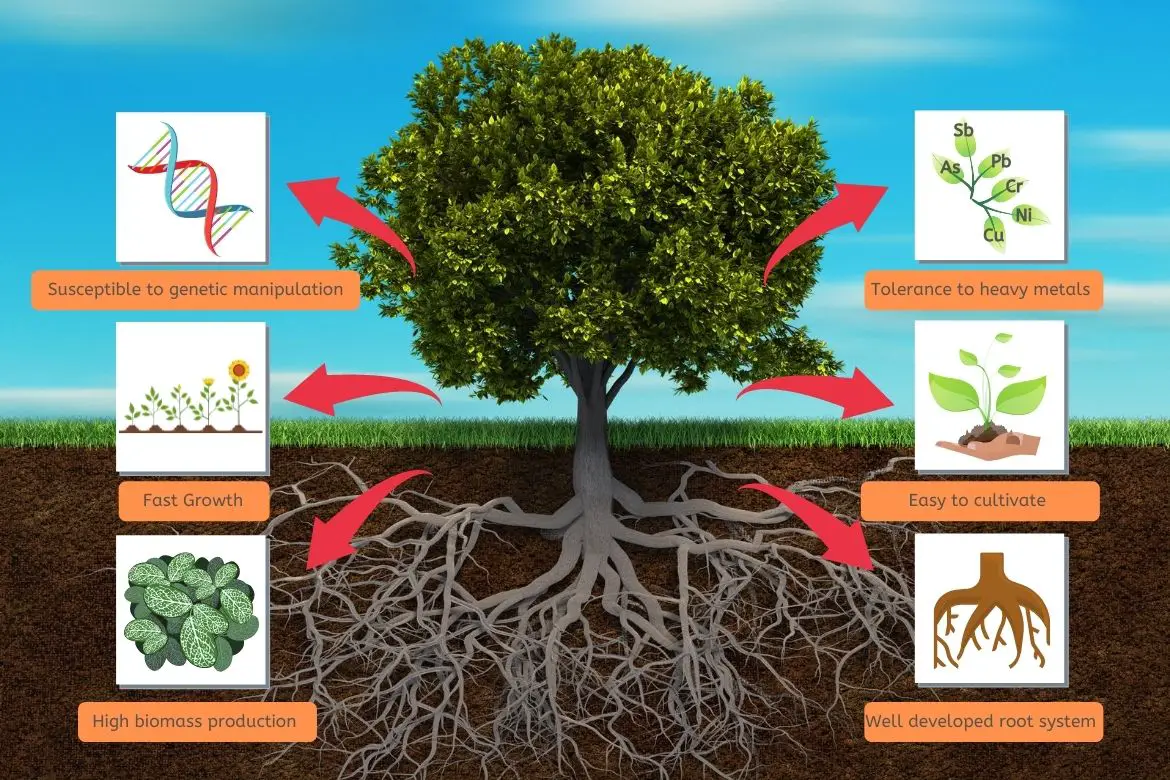
Utilizing plants for remediation of a contaminated site involves several mechanisms.
Root systems play a key role in preventing toxicity induced by contaminants. Pollutants are absorbed by plants via the root system.
In order to allow absorption and accumulation of water and nutrients, a sufficient surface area is provided by the rhizosphere.
Similarly, researchers are examining whether trees can be used to effectively remove pollutants from deeper soil layers that are easier to penetrate by the deeper roots of trees compared to small plants.
By releasing inorganic and organic compounds (exudates) into the rhizosphere, plant roots also modify the soil-root interface.
As a result of root exudates, microorganisms multiply, soil particles become more stable, and contaminants become more readily bioavailable.
Plant exudates affect the mobility and bioavailability of pollutants in the rhizosphere by modifying soil characteristics, changing chemical composition, releasing organic compounds and increasing plant-assisted microbial activity.
Further in the article, advantages and disadvantages of phytoremediation are discussed.
Advantages of Phytoremediation
The use of plants provides numerous advantages over conventional remediation methods
- In soil and water, this technology is an effective, efficient, and environmentally friendly means of degrading, diminishing, or detoxifying contaminants.
- Due to its low maintenance and harvesting costs, it is an inexpensive method.
- It is a carbon-neutral technology since it does not result in any additional carbon dioxide emissions beyond the ranges originally incorporated upon the combustion of plant biomass
- Social acceptance is high and it is aesthetically pleasing.
- Aside from tackling the problem of removing toxicants from the environment, this technique can also be used to recover commercially important metals from the soil.
- Plant biomass can also be used to generate energy, making it a lucrative technology.
- A technique such as this can be used to remediate metals, solvents, pesticides, crude oil, poly aromatic hydrocarbons, landfill leachate, and explosives.
Disadvantages of Phytoremediation
- Since high levels of toxicity pollution are induced in plant cells, hindering plant growth, this technique can best be utilized on sites with low or moderate levels of contamination.
- The process is extremely time-consuming.
- Hyperaccumulator plant species have limited ability to take up contaminants from the soil because of their low biomass and slow growth rate.
- A mismanaged process can set the stage for the entry of toxic compounds into the food chain.
- Metal ions tightly bound have a limited bioavailability, limiting their removal.
2 comments
Good post. I learn something new and challenging on sites
I stumbleupon on a daily basis. It’s always exciting
to read through articles from other authors and practice
a little something frokm their websites.
Thank you, your site is very useful!