Table of Contents
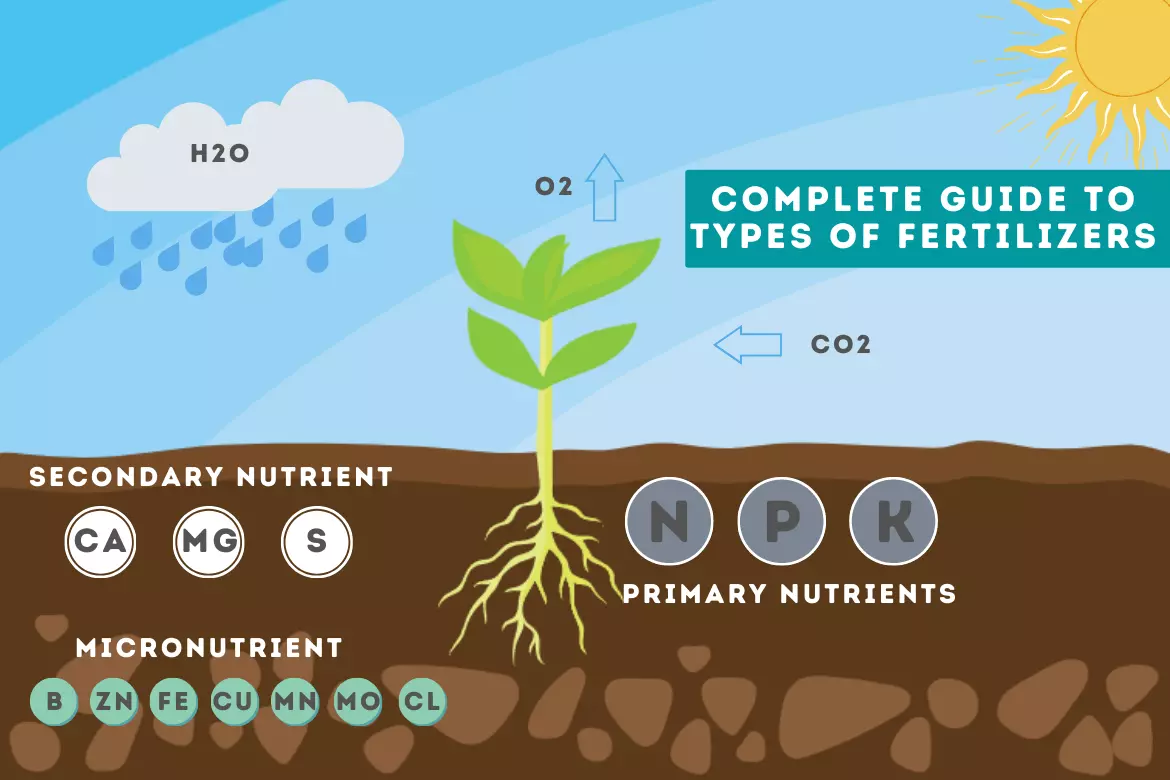
Essential Nutrients for plants
Role of availability of essential nutrients is vital for good crop health and higher crop yields.
| Essential nutrients for plants | |
|---|---|
| Primary Nutrients | Nitrogen |
| Phosphorus | |
| Potassium | |
| Secondary Nutrients | Calcium |
| Magnesium | |
| Sulphur | |
| Micronutrients | Boron |
| Chlorine | |
| Copper | |
| Iron | |
| Manganese | |
| Molybdenum | |
| Zinc | |
With the advancement of technology and science, humans have figured out many ways to supplement the soil with the above mentioned essential nutrients in the soil. Inorganic fertilizers and biofertilizers have played a crucial role in agriculture.
*A must read : What is bio fertilizer: Its Types and Uses
Function of nutrients in plants
| Nutrient | Function | Availability to plant | Symbol |
|---|---|---|---|
| Nitrogen | Promotes rapid growth Chlorophyll formation and protein synthesis | Anion and Cation | NO3- NH4+ |
| Phosphorus | Stimulates early root growth Hastens maturity Stimulates blooming Aids seed formation | Anion | H2PO4- HPO4- - |
| Potassium | Increases resistance to drought and disease Increases stalk and straw strength Increases quality of grain and seed | Cation | K+ |
| Calcium | Improves root formation Stiffness of straw and vigor Increases resistance to seedling diseases | Cation | Ca++ |
| Magnesium | Aids chlorophyll formation and phosphorus metabolism Helps regulate uptake of other nutrients | Cation | Mg++ |
| Sulfur | Amino acids Vitamins Imparts dark green color Stimulates seed production | Anion | SO4- - |
| Boron | Aids carbohydrate transport and cell division | Anion | H3BO3 H2BO3- HBO3- - BO3- - - B4O7- - |
| Copper | Enzymes Light reactions | Cation | Cu++ |
| Iron | Chlorophyll formation | Cation | Fe++ Fe+++ |
| Manganese | Oxidation-reduction reactions. Hastens germination and maturation | Cation | Mn++ |
| Zinc | Auxins Enzymes | Cation | Zn++ |
| Molybdenum | Aids nitrogen fixation and nitrate assimilation | Anion | MoO4- - |
| Cobalt | Essential for nitrogen fixation | Cation | Co++ |
| Nickel | Grain filling, seed viability | Cation | Ni++ Ni+++ |
| Chlorine | Water use | Anion | CI- |
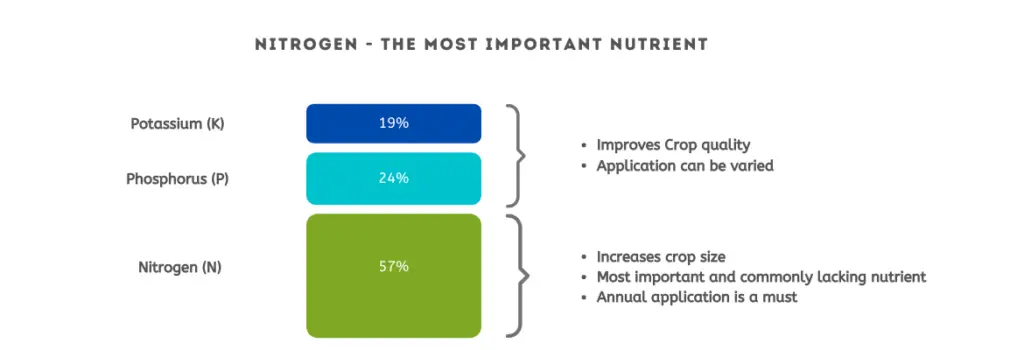
Inorganic Fertilizers
Inorganic fertilizer is any substance of synthetic origin that is added to the soil to provide nutrients to plants.
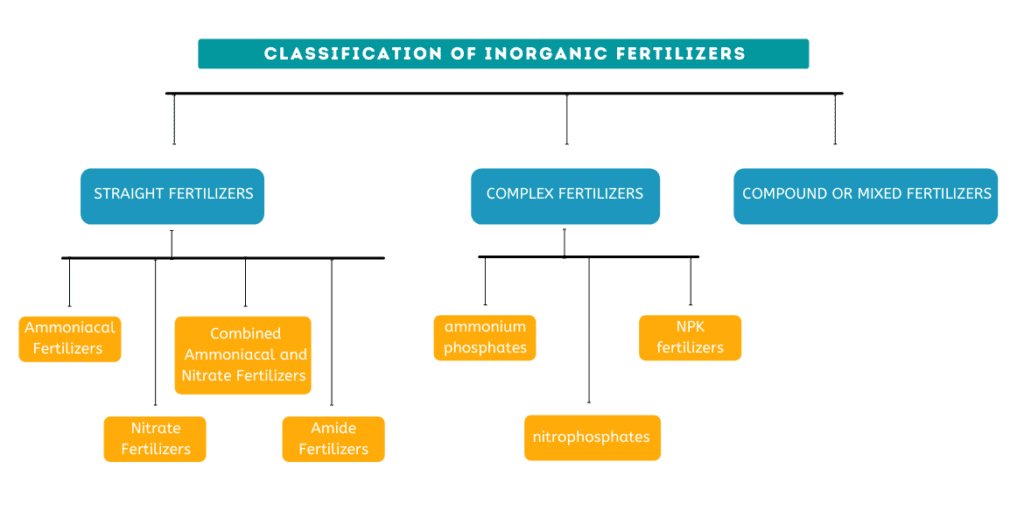
Classification of inorganic fertilizer
Inorganic fertilizers can be classified into three categories:
(i) Straight fertilizers : Fertilizers that supply only one primary chemical element are called straight fertilizers.
(ii) Complex fertilizers : Complex fertilizers are multi-nutrient fertilizers produced by chemical reactions between components that contain the primary plant nutrients. The individual granules produced by the chemical reactions have all the nutrients intended.
(iii) Compound fertilizers OR Mixed fertilizers : As the name implies, compound or mixed fertilizers consist of granules or blends of different single-nutrient fertilizers. These are physical mixtures of straight fertilizers. The individual granules still have only one nutrient.
Fertilizers can also be classified on the basis of physical form i.e. Solid or Liquid.
Straight Fertilizers
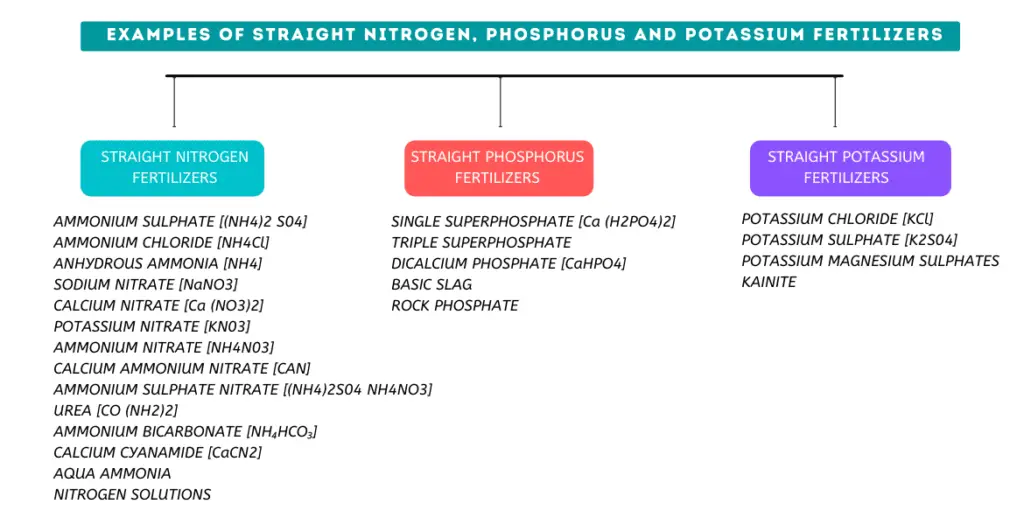
Nitrogen straight Fertilizers
Classification of straight Nitrogen fertilizer
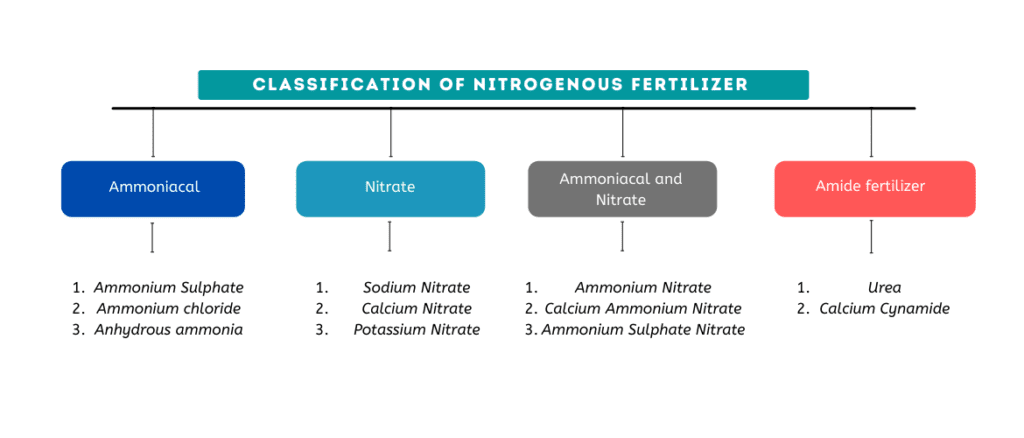
There are four basic types of nitrogen fertilizers based on the chemical form in which the nitrogen is available: ammonium compounds, nitrate compounds, combined ammonium compound and nitrate compounds, and amide compounds.
(i) Ammoniacal Fertilizers
Ammoniacal fertilizers contain nitrogen in the form of ammonium ions, NH4 +. Ammonium ions are not lost to leaching in soil because they are adsorbed by soil colloids, but they are rapidly converted to nitrate by bacteria. Crops are able to take up some of their nitrogen as ammonium ions during the early stages of growth, so ammoniacal fertilizers provide adequate nitrogen either before or after nitrification.
Continuous application of ammoniacal fertilizers can increase soil acidity. Ammonium sulfate and ammonium chloride are examples of ammoniacal fertilizers.
(ii) Nitrate Fertilizers
The nitrogen in nitrate fertilizers is in the form of the nitrate ion, NO. Plants absorb a large proportion of their nitrogen in this form. It is not possible for soil colloids to retain nitrate fertilizer. As a result, nitrogen will be lost by leaching if the application of nitrate fertilizers is followed by heavy rains or irrigation.
It is also common for nitrates to undergo denitrification particularly in waterlogged soils, so they are generally not recommended for wetland rice. When applied to soil, nitrate fertilizers have an alkaline effect. Examples include sodium nitrate and calcium nitrate.
(iii) Combined Ammoniacal and Nitrate Fertilizers
Both ammoniacal and nitrate ions are present in these fertilizers. So, they have the advantages and disadvantages of both ammoniacal and nitrate fertilizers. Ammonium nitrate, ammonium sulphate nitrate, and calcium ammonium nitrate are examples of common straight fertilizers of this type.
(iv) Amide Fertilizers
The nitrogen in these simple organic compounds is not readily available to plants. An amide fertilizer is rapidly converted into an ammoniacal form and then to nitrate form when applied to the soil. As they are soluble in water, care must be taken when applying them to the soil to prevent nitrogen loss through leaching. One of the most significant examples of amide fertilizer is urea.
Examples of straight nitrogen fertilizers
Following are the examples of nitrogen fertilizers commonly used in agriculture
AMMONIUM SULPHATE [(NH4)2 S04]
One of the earliest synthetic nitrogenous fertilizers (has an appearance like white salt) was ammonium sulphate (20.7 per cent nitrogen and 24.0 per cent sulfur). However, because of its inferior nutrient content and relatively high manufacturing costs, its significance has diminished and it has been largely replaced by fertilizers with a higher concentration of nitrogen.
In addition to being applied prior to sowing, ammonium sulfate can be applied to the soil as a top dressing when a plant begins to grow.
Its sulphur content makes it a particularly useful nitrogen fertilizer in areas where sulphur is deficient.
Mixing with seeds should be avoided since germination may be affected.
Due to the soil colloids retaining the ammonium nitrogen in this fertilizer, and the subsequent resistance to leaching, it is an excellent fertilizer for wetland rice cultivation and jute cultivation.
It is easy to handle and stores well when kept dry. It can sometimes form lumps during the rainy season.
Sulphide injury can occur if ammonium sulphate is used in highly reduced conditions or on acid-sulphate soils.
Ammonium sulphate has an acidifying effect. Thus, its continuous use may increase soil acidity and lower crop yields (though it can be beneficial on alkaline soils). Calcium carbonate (limestone) can offset the acidifying effect of ammonium sulphate; 110 kg of calcium carbonate can offset 100 kg of ammonium sulphate.
AMMONIUM CHLORIDE [NH4Cl]
Ammonium chloride is either produced by neutralizing ammonia with hydrochloric acid or as a by-product of the manufacture of soda ash. There are very few countries where these products are made, and small amounts are produced.
Ammonium chloride is white and crystalline in nature and it contains 25-26 percent nitrogen. Physically, it is similar to ammonium sulfate and is soluble in water.
In the same way as ammonium sulphate, ammonium chloride can be applied prior to sowing, and as a side and top dressing when the crop is growing.
Ammonium chloride is more acidic than ammonium sulphate, requiring 128 kg of calcium carbonate to neutralize 100 kg of ammonium chloride.
Adding ammonium chloride may also result in greater calcium losses due to its conversion to soluble calcium chloride that is easily leached out.
Ammonium chloride is generally rated as equal to ammonium sulphate and other nitrogen fertilizers in terms of its agronomic suitability. However, tobacco, vegetables like tomato, potatoes, celery, asparagus, onion, cucumber, lettuce, broad beans and fruits like gooseberry, raspberry, strawberry, blackberry, blueberry, mango, avocado, peach, pomegranate and a number of other crops sensitive to chloride are not recommended to be treated with ammonium chloride.
ANHYDROUS AMMONIA [NH4]
At normal temperatures and atmospheric pressure, ammonia (82 percent nitrogen) is a colourless, pungent and toxic gas. Liquidization can be achieved by cooling or applying pressure, and the gas is handled as a liquid in storage and transportation.
Anhydrous ammonia is normally not explosive, but when mixed with air in some proportions it may ignite by a spark; the presence of oil increases the explosion risk.
Anhydrous ammonia can be injected directly into the soil using pressurized equipment, using a special line that applies it 10-20 cm below the surface due to its volatile nature.
Anhydrous ammonia is as effective as most solid nitrogenous fertilizers agronomically. It must, however, be handled, stored, transported and used with special equipment and care.
The equipment used in anhydrous ammonia applications is expensive and quite sophisticated, hence it is only suitable for large agricultural and contracting operations.
SODIUM NITRATE [NaNO3]
Soda ash is treated with nitric acid to make synthetic sodium nitrate.
For acidic soils, sodium nitrate is particularly useful.
Sodium nitrate is a crystalline white substance that is highly soluble in water.
The nitrate ions are absorbed by the plants when sodium nitrate is applied to the soil. Sodium nitrate applied to the soil for long term adversely affects the soil structure because of the accumulation of the left out sodium ions which are not absorbed by the plants.
Because of its low nutrient content (16 per cent Nitrogen), its use as a nitrogen fertilizer is limited, and because of the risk of nitrate leaching, it is preferably applied to actively growing crops.
Chile salt peter or Chilean nitrate are other names for sodium nitrate, as it occurs naturally in Chile. Chile is one of the largest producers of this substance.
CALCIUM NITRATE [Ca (NO3)2]
Crushed limestone reacts with nitric acid to produce calcium nitrate. This compound can also be produced by some nitrophosphate (complex fertilizer) processes as a by-product.
Calcium nitrate is granular in form and almost white in colour. It is extremely hygroscopic, highly alkaline in reaction, and highly soluble in water.
It contains almost 15.5 per cent of nitrogen and 19.5 per cent of calcium.
It is considered to be an excellent source of nitrogen for a number of vegetable and fruit crops that require calcium specifically. Furthermore, calcium also assists in maintaining the soil pH.
As with sodium nitrate, calcium nitrate is preferably applied when crop growth is active to avoid leaching and loss.
Because of its low concentration, its use as a fertilizer is limited.
POTASSIUM NITRATE [KN03]
Purified potassium nitrate contains 13.0% nitrogen and 36.4% potassium.
Potassium nitrate is a powerful oxidizer. Potassium nitrate colour ranges from white to dirty grey crystalline solid. It is water-soluble.
Potassium nitrate fertilizer is preferred for growing conditions where a highly soluble, chloride-free nutrient source is needed. All of the available N is immediately available for plant uptake as nitrate in such soils, requiring no further microbial action or soil transformation.
A potassium nitrate application to the soil is made before the growing season or as a supplement during the growing of the crop.
In addition, it is commonly used for polyhouse plant production and hydroponic culture.
AMMONIUM NITRATE [NH4N03]
Ammonium nitrate is a white powder, but fertilizer grades are granular or prilled. This compound contains 33-34.5 per cent nitrogen, is highly soluble in water, and is hygroscopic.
As long as moisture is not picked up by packaging or storage conditions, ammonium nitrate pellets are free-flowing and do not present a problem for handling and storage.
When combined with combustible materials, ammonium nitrate can be a fire and explosive hazard. Care should be taken to comply with codes of practice for handling, transport, and storage.
Ammonium nitrate can be applied before the crop is planted or as a side or top dressing. Ammonium nitrate is ideal for most crops, except for wetland rice, since it contains both ammoniacal and nitrate nitrogen.
Since it contains nitrogen in half ammonium and half nitrate form, it is, overall, intermediate in leaching propensity compared to ammoniacal or nitrate fertilizers.
While soils tend to become acidic, the acidifying effect is less when compared to ammonium sulphate. 59 kg of limestone is needed to offset the effect of 100 kg of ammonium nitrate.
CALCIUM AMMONIUM NITRATE [CAN]
Calcium ammonium nitrate (CAN) is formed by diluting ammonium nitrate with a non-reactive material, usually limestone, to reduce the hazards associated with its use of ammonium nitrate as a stand-alone substance.
Pulverized limestone or dolomite is granulated with concentrated ammonium nitrate solution to produce calcium ammonium nitrate.
To prevent moisture from picking up and caking, the granules are coated with an inactive dust after cooling. (Depending on the coating dust) CAN granules are light grey to light brown in colour and free-flowing.
Under humid tropical conditions, CAN poses storage problems, so it is stored in air-conditioned silos.
Half of the nitrogen in commercial CAN comes in the form of ammonia and half is in the form of nitrate. CAN contains 25-28 per cent nitrogen.
Like ammonium nitrate, it has similar agronomic characteristics. However, CAN is relatively neutral in its reaction when applied to soil, unlike ammonium nitrate. it can even be applied to acidic soil.
AMMONIUM SULPHATE NITRATE [(NH4)2S04 NH4NO3]
The double salt of ammonium sulphate and ammonium nitrate is ammonium sulphate nitrate (ASN). About 62.5 per cent of it is ammonium sulphate, 37.5 per cent is ammonium nitrate, and contains 26 per cent nitrogen and 12.1 per cent sulphur.
ASN can be crystalline or granular. Crystalline form is white, but granular form takes on the colour of any protective coating dust applied.
Its 100% water-soluble and no residue is left behind after it is dissolved in water.
75 percent of the nitrogen is in the form of ammonium, and 25 percent is in the form of nitrate. In addition to nitrogen, it also supplies sulphur.
Crystalline ASN can cake in storage and must be broken up before being used.
An ASN application can be applied prior to sowing, during sowing, or as a side or top dressing.
A mixed ammoniacal or nitrate source of nitrogen, it has a slightly lower leaching risk than ammonium nitrate.
An acidic effect is produced by ASN, which is intermediate between ammonium sulphate and ammonium nitrate – 85 kg of limestone are needed to neutralize the effects of 100 kg of ASN.
UREA [CO (NH2)2]
As the most concentrated solid nitrogen fertilizer, urea has noticeable advantages in storage, transport, and handling. It is widely available on the market, and it often costs less per unit of nitrogen than other nitrogen fertilizers. Thus, its use is increasing rapidly on a global scale.
The granules or prills of urea are white and free-flowing. Due to the urea’s hygroscopic nature, appropriate packaging is needed to reduce moisture contamination. Commercial urea contains 46 percent nitrogen, in the form of amide.
Urea is converted to ammonium carbonate as soon as it is applied to the soil, leading to high concentration of ammonia in the soil.
The ammonia is held by the colloids of the soil when urea is mixed with t soil. But when urea is applied on the surface level of the soil, then majority of ammonia might get lost in the atmosphere due to volatilization. The amount of ammonia being lost depends on the type of the soil, soil moisture and temperature and rainfall.
Additionally, urea damages young seedlings. Thus urea should be used carefully. Urea is highly soluble in water. Hence, it is advisable to use it in solution fertilizers or foliar sprays.
It has acidic reaction in the soil. 80 Kg of limestone will be able to offset the acidic effect of 100 Kg of urea.
Biuret, a toxic impurity, is sometimes found in urea. As urea is heated to above 140°-170°c, two molecules of urea become biuret as the NH3 is eluted. If water or ammonia are present, more biuret is formed. Biuret toxicity has been reported for multiple crops. Biuret-rich urea has been shown to adversely affect the germination and growth of wheat and maize seeds.
When urea is applied in a band in proximity to the seed, the germination of wheat and barley seeds is affected even if biuret content in urea is 1-2 per cent. However, in broadcast method of application, urea with even 10% of biuret doesn’t have any adverse effect. If urea is sprayed, the biuret content should not exceed 1 per cent.
Biuret content in urea causes yellowing of leaves and cupped growth in citrus, coffee and pineapple. Biuret also affects the metabolism of proteins as well as causes proteolysis. Plants have been observed to have biuret for months. Therefore, commercial urea is checked and quality controlled so that biuret content can be kept below danger levels.
Urea is suitable for most crops and can be used on all types of soils and can be applied at sowing or as a top dressing.
AMMONIUM BICARBONATE [NH₄HCO₃]
Some Asian countries, particularly China, use ammonium bicarbonate to a limited extent.
The nitrogen content of ammonium bicarbonarte fertilizer is 17 per cent.
It may lose some of its ammonia to the atmosphere before it can be absorbed by the soil due to its instability, particularly when applied as top dressing to calcareous or alkaline soils.
CALCIUM CYANAMIDE [CaCN2]
This is a calcium salt of the cyanamide (CN 2− 2. ) anion. Calcium cyanamide is also known as nitrolime. It contains 21 per cent of nitrogen.
It is a greyish-white powdery substance that decomposes in moist soil, producing ammonia.
In addition to acting as a nitrogen fertilizer, it also kills insects, soil parasites, and harmful fungi, and hence also functions as an effective pesticide and fungicide.
Also, Calcium cyanamide works as a defoliant and herbicide by preventing weed germination.
AQUA AMMONIA
In most cases, aqua ammonia (ammonia dissolved in water) contains 20 percent nitrogen, although it can contain up to 26 per cent nitrogen in some commercial grades.
Aqua ammonia offers many advantages over anhydrous ammonia, including its simpler handling requirements and its non-pressurized nature, which eliminates most hazards.
Storage of aqua ammonia can be achieved with ordinary storage tanks as opposed to stainless steel storage tanks in the case of Anhydrous ammonia, which can be costly.
Aqua ammonia must also be applied deep into the soil to prevent nitrogen loss.
NITROGEN SOLUTIONS
There are two types of nitrogen solutions: non-pressure and low-pressure.
Usually, non-pressure solutions are produced from urea and ammonium nitrate and contain up to 28-32 percent nitrogen. Pressurized solutions are made by combining ammonia with ammonium nitrate or urea or both, and may contain as much as 41 percent nitrogen.
Its advantage is that it has a higher nutrient content than non-pressurized solutions, but it is expensive because of the need for pressurization, distribution and application equipment.
Phosphorus straight Fertilizers
Phosphorus is an important component of the earth’s crust, but has been concentrated over geological time in deposits of phosphate rock (formed mostly from aquatic organism remains). It is present in most natural and cultivated soils in insufficient amounts for full crop growth.
In order for fertilizer phosphorus to be available to plants, it must be released in ionic form to the soil solution. Plants absorb phosphorus from the soil solution as phosphate ions (HPO4 and H2PO4). Fertilizers contain phosphorus in a variety of chemical and physical forms, whose availability varies greatly.
Examples of straight Phosphorus fertilizers
Simple superphosphate, concentrated superphosphate, slag and rock phosphate are some of the straight phosphorus fertilizers typically used. A brief description of these fertilizers is given below.
SINGLE SUPERPHOSPHATE [Ca (H2PO4)2]
The first chemically manufactured phosphorus fertilizer was single (normal) superphosphate (SSP). Although it is still used in many countries, it has been and is being replaced by more concentrated phosphorus fertilizers and by complex fertilizers.
It is made In a specially designed reaction vessel, finely ground rock phosphate is mixed with concentrated sulphuric acid. Multinutrient fertilizers are made by drying and granulating the product, sometimes with nitrogen and potassium fertilizers.
SSP is grey or brown, typically granular for ease of storage and application. The powdered product cakes in storage. In the field, SSP granulated can be applied easily and evenly without a problem.
Almost equal quantities of monocalcium phosphate and calcium sulfate (gypsum) are present in SSP. It typically contains 17-20 percent total P205, of which over 90 percent is water-soluble; it also contains about 16 percent sulfur.
There is a small amount of free acid in this SSP, so packaging should be able to prevent an acid attack. Good packaging material can be polyethylene or polyethylene-lined sacks.
SSP is a suitable phosphorus fertilizer for most crops and soils, with the exception of highly acidic soils, where water-insoluble phosphate sources, such as rock phosphate, are more suitable.
A soil that lacks calcium and sulphur will benefit from the calcium and sulphur SSP contains.
When the fertilizer is applied in bands close to the row of seeds, the water-soluble phosphate contained in SSP will be immobilized in the soil more slowly. This will minimize soil-fertilizer contact.
TRIPLE SUPERPHOSPHATE
TSP (triple superphosphate) is made by reacting finely ground phosphate rock with concentrated phosphoric acid (52 to 54 per cent P2O5). The granules are usually granulated as a stand-alone fertilizer or as a component of multi-nutrient fertilizers.
TSP contains P2O5 in the range of 44 to 52 per cent, and is almost completely water-soluble.
Powdered TSP tends to cake, but granulated TSP has excellent storage and handling properties and is free-flowing. As TSP may contain free phosphoric acid, suitable packaging is required.
As phosphorus fertilizers, TSP and SSP serve similar purposes, with the difference that TSP has a far higher concentration of nutrients and has much less sulfur. Since it contains a high amount of nutrients, it is particularly useful for preparing high-quality multinutrient fertilizers.
DICALCIUM PHOSPHATE [CaHPO4]
As a fertilizer, straight dicalcium phosphate is seldom used because of the high costs of manufacture and the inconvenient handling and application of its powder form. It is made by reacting rock phosphate with hydrochloric acid and adding lime to produce a precipitate.
The commercial product contains about 35 percent P205, a citrate-soluble but water-insoluble compound. Dicalcium phosphate is also a citrate soluble ingredient of nitrophosphates and other compound fertilizers.
Because dicalcium phosphate’s citrate-soluble phosphate does not undergo soil immobilization as quickly as monocalcium phosphate, it is considered to be an effective fertilizer phosphorus source for long-term crops such as sugarcane or crops of acidic soils.
BASIC SLAG
Basic slag is a by-product of the steel industry. However, the amount of phosphate-rich slag has been declining as the steel industry is adopting modern technologies and also the ores used to manufacture steel.
During the manufacture of steel, non-ferrous elements, including phosphorus, from the ore are separated from the ore as slag along with the residues of lime added during the manufacturing process.
The slag may contain up to 18 per cent P205, and it also has considerable liming value.
Basic slag contains wáter-insoluble but citric-acid soluble phosphate in the form of calcium silicophosphates; it is unstable and becomes available slowly, particularly in acid soils. Slag is therefore best suited for long-duration crops, especially on acidic soils; it can also provide phosphorus to neutral and slightly acidic soils.
For the optimum release of phosphorus into the soil solution, basic slag must be finely ground.
Basic slag is not hygroscopic and stores well, but powder application can be very dusty; it may also be difficult to achieve uniform application.
ROCK PHOSPHATE
In order to increase soil contact and dissolution, rock phosphate is finely ground for direct application. Typically, it is advised that the fineness of the grinding should be such that 90 per cent of the rock phosphate should pass through a 100 mesh sieve.
The suitability of rock phosphate for direct application varies from source to source, with those from Tunisia and Morocco being the best.
Finely ground rock phosphate is light grey or brown in colour and neutral in nature. The phosphorus content of rock phosphate is in the range of 29 to 37 per cent P2O5.
Rock phosphate is a slow-acting phosphorus fertilizer.
The calcium content of rock phosphate ranges from 35 to 38 per cent, however, there is no liming value to it.
Phosphorus in ground rock phosphate is generally utilized best in soils with a pH below 5.5 or in soils rich in organic matter. On neutral or alkaline soils, phosphorus from rock phosphate is almost unavailable to crops.
The capacity of crops to use rock phosphate for phosphorus varies somewhat depending on the soil type on which they are grown. Turnips, sweet clover, mustard, tea, rubber, and coffee are the most efficient users of rock phosphate, while cotton, rice, wheat, barley, and potatoes are the least efficient.
It is essential to maximize contact with the soil, so rock phosphates should be broadcast, not placed. Application of rock phosphate before sowing the seeds also gives some time for solubilization to take place.
Potassium straight Fertilizers
Potassium (K) is an essential nutrient for plant growth. The potassium content of fertilizers is commonly expressed in terms of potassium oxide (K2O) or “potash”.
Potassium fertilizers are mined and purified from natural deposits containing potassium salts found in various countries, including Cavada, the United States, the former Soviet Union, France, Germany, and Spain. SOme of the key potassium minerals are sylvinite (a mixture of sylvite (KCl) and halite (NaCl)), carnallite (KC1.MgCl2: :6H2O), kainite (KCl.MgSO4.3H2O), langbeinite (K2SO4.2MgSO4,) and nitre (KNO3).
Examples of straight Potassium fertilizers
Common potassium fertilizers are potassium chloride (muriate of potash), potassium sulphate (sulphate of potash), and potassium magnesium sulphate.
POTASSIUM CHLORIDE [KCl]
Potassium chloride contains about 60% potash (K2O).
Potassium chloride is a crystalline white salt, but the colour of fertilizer grade potassium chloride ranges from white to red depending on the amount of impurities in the potash minerals. The colour does not affect the fertilizer effect.
The crystallized potassium chloride is free-flowing and does not pose any problems in handling and storing. Formally, the fertilizer used to cake up, but this problem can be removed by mixing anti-caking agents.
The potassium chloride salt is 100% soluble in water. When applied to soil, the potassium ion is adsorbed and retained by soil colloids, so there is little possibility of leaching. Plant roots take up the ionic form of the nutrient.
Potassium chloride is neutral in nature and does not produce acidity or alkalinity in the soil.
The chlorine content of potassium chloride is about 47 per cent.
Although potassium chloride is suitable for most crops and soils, potassium sulfate is preferred for crops such as tobacco and potatoes, where excess chloride affects quality.
Generally, the entire potassium requirement can be applied as a basal dose, but in sandy soils, high rainfall areas, and wetland rice, a split application is preferred.
POTASSIUM SULPHATE [K2S04]
The most common potassium fertilizer is potassium chloride, but potassium sulphate is used to a lesser extent for specific crops.
In nature, potassium sulphate occurs as langbeinite, a double salt with magnesium (K2SO4.2MgSO4), but it can also be manufactured by the action of sulfuric acid on potassium chloride.
White crystalline salt, potassium sulphate is free-flowing and contains 48 to 52 per cent potash (K2O) and 18 per cent sulfur. Normally, handling and storing crystalline potassium sulfate does not pose any problems.
Potassium sulphate is soluble in water, and when applied to the soil, the potassium ions are retained by soil colloids and do not easily leach out.
It is an excellent fertilizer that can be applied to all soil types and crops. However, since it is more expensive, it is usually used only in cultivating chloride-sensitive crops.
Potassium sulphate is soluble in water, and when applied to the soil, the potassium ions are retained by soil colloids and do not easily leach out. It is an excellent fertilizer that can be applied to all soil types and crops.
Due to its sulphur content, it is a two-nutrient fertilizer. It can be used for tobacco, potatoes, fruits and vegetables.
Additionally, it may be a good choice for saline soils as well as in poly house where chloride accumulation can be a problem.
POTASSIUM MAGNESIUM SULPHATES
There are several fertilizers that contain both potassium and magnesium in the sulphate form, such as the above-mentioned langbeinite or schoenite (K2S04.MgSO4.6H20).
Potassium magnesium sulphate is commercially produced in Europe and the United States.
Potassium magnesium sulphate has 22-30 per cent K2O, 10-19 per cent MgO, 16-23 per cent Sulphur.
The use of potassium magnesium sulphate is especially recommended for acidic soils and soils deficient in magnesium. Additionally, it is recommended for crops with high magnesium requirements, such as potatoes, fruits, vegetables, and forest trees.
KAINITE
Kainite is a naturally occurring mineral.
Pure kainite has the chemical composition kcl.MgSO4.3H2O, but in nature, it rarely occurs as such.
Kainite, a commercially available product, is largely composed of potassium chloride, magnesium sulphate, and magnesium and sodium chlorides.
Kainite contains 14-22 percent K2O.
It is alkaline in nature and contains 46 per cent chlorine.
Unlike most other potassic fertilizers, it may cake in storage and need to be broken up before use.
It can be beneficial for crops that use sodium, such as sugarbeet.
Complex fertilizers
As a result of the high nutrient content of complex fertilizers, the cost of packing, handling, and transport per unit of nutrient is lower than that of many straight fertilizers.
Complex fertilizers are available in granular form and are free-flowing, making them easy to handle and apply.
Complex fertilizers have the advantage of ensuring balanced fertilization of crops, especially in developing countries. The production and use of complex fertilizers is therefore on the rise and accounts for a considerable proportion of world fertilizer consumption.
Classification of complex fertilizers
Complex fertilizers can broadly be classified into (I)ammonium phosphates, (II)nitrophosphates and (III)NPK fertilizers.
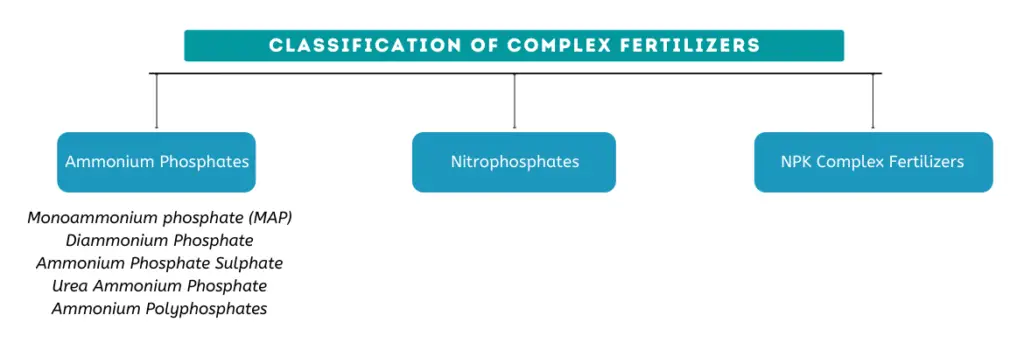
Examples of complex fertilizers
I. Ammonium Phosphates
In general, ammonium phosphates are satisfactory for all crops and soils. It exhibits the characteristics of nitrogen fertilizers containing ammonium as well as highly water-soluble phosphate.
It is possible that, in some circumstances, nitrogen from urea ammonium phosphate will be less effective.
Crops are not immediately able to utilize the polyphosphate in ammonium polyphosphates, however, it is quickly transformed to the available orthophosphate form in soil.
Due to its high phosphorus content, DAP is used more extensively and in crops where the phosphate requirement is relatively high; on the other hand, MAP is usually mixed with additional nitrogen and potassium intermediates due to its wide N:P205 ratio.
(i) Monoammonium phosphate (MAP)
Monoammonium phosphate (MAP) is a high-analysis fertilizer that is almost completely soluble in water. It contains 52 to 55 per cent P2O5 and 11 to 12 per cent nitrogen.
Because it is non-hygroscopic and compatible with most other fertilizer materials, it is widely used in the manufacture of multi-nutrient fertilizers. Produced by reacting ammonia with wet phosphoric acid at a concentration of 45-52%, maintaining an NH3:H3PO4 ratio of 1:1.
Spray-drying of the concentrated MAP solution yields powdered material which is later granulated for application in the fields.
(ii) Diammonium Phosphate
DAP (diammonium phosphate) is produced in large quantities. Commercial DAP is mostly water-soluble, free flowing and granular and contains 18 per cent nitrogen and 46 per cent P2O5.
The manufacturing process of diammonium phosphate requires a mole ratio of 2:1 between NH3 and H3PO4, which involves an additional step of ammoniation.
The slurry thus produced is granulated, dried, screened, cooled and conditioned by a coating agent.
(iii) Ammonium Phosphate Sulphate
Approximately 60% of ammonium phosphate sulphate is ammonium phosphate, while 40% is ammonium sulphate. It contains 16 percent nitrogen and about 20% P2O5.
Nitrogen content can be increased by adding urea, and a variety of N:P2O5 analysis products can be obtained.
Ammonium phosphate sulphate is a free-flowing substance that is usually not difficult to handle and store.
(iv) Urea Ammonium Phosphate
The chemical reaction between ammonia and phosphoric acid produces urea ammonium phosphate (UAP).
In the granulator, additional ammonia and urea are added to the ammonium phosphate slurry. A coating agent is applied to prevent caking after the material has been dried, screened and cooled.
There are various N:P2O5 analyses available. Also, it is possible to produce liquid (solution) UAP directly, thereby avoiding drying costs.
Almost all the phosphorus is water-soluble, while some nitrogen is in the form of ammoniacal and some in the form of urea.
Free-flowing granules and good physical properties make the fertilizer an excellent choice for soil, although it may cake when humid.
(v) Ammonium Polyphosphates
By reacting ammonia with superphosphoric acid, ammonium polyphosphates (APP) are produced. Both liquids and solids are made of them.
The typical APP solutions in the USA have analyses of 11-33-0, 10-34-0, 12-40-0, and 8-27-0; however, granular products can be produced with nutrient contents of up to 15-61-0, depending on the acid purity used. APP is completely soluble in water.
In APP, nitrogen is entirely in the form of ammoniacal nitrogen, and phosphate is present as monoammonium phosphate (NH4H2PO4) and orthoammonium polyphosphates.
In addition to their high analysis, APP solutions allow for the addition of large quantities of micronutrients without precipitation. Ammonium polyphosphates are mainly manufactured and used in the United States.
II. Nitrophosphates
Nitrophosphates are fertilizers made by nitrifying phosphate rock with nitric acid or a mixture of nitric and sulphuric acids, followed by ammoniating the resulting slurry. Afterwards, the slurry is granulated or prilled. Additional nitrogen can then be added, in the form of ammonium nitrate, along with potassium chloride or sulphate, to achieve the desired NPK analysis.
Granulation characteristics of nitrogen phosphates are good, and they are coated to minimize moisture absorption. When properly packaged and stored, cakes do not form.
Solubility of the phosphate determines the agronomic performance of nitrophosphates. Most phosphate is citrate-soluble, however, its solubility in water varies (0-80%) based on the ammoniation process.
In general, all crops and soils are suitable for nitrophosphates containing 60 per cent or more water-soluble phosphate. However, low water-solubility phosphates are suitable only for long duration crops such as sugarcane or grassland, and for acid soils.
Short duration crops like cereals and potatoes are less suitable for Nitrophosphates.
III. NPK Complex Fertilizers
Nitrogen, phosphorus, and potassium are contained in varying proportions in solid NPK fertilizers. Generally, they are easy to handle and apply, free flowing and granular in structure. Various grades are produced and marketed depending on soil and crop needs.
They can be prepared either by the ammonium phosphate or nitrophosphate routes by adding potassium. The production process used determines the ratio of ammonium, nitrate and urea nitrogen. The production process also determines the the content of water-soluble and citrate-soluble phosphorus.
The best way to apply them is as a basal dressing. In spite of the extensively wide range of available NPK analyses, most factories limit their output to a few products for operational reasons.
The main benefit of NPK complex fertilizers is their ease of use, including ease of handling and application of all three nutrients in just one operation. Additionally, they can include calcium, magnesium, phosphorus, and micronutrients.
There may, however, be some situations where the farmer might need to apply additional amounts of these nutrients separately, as the available grades of NPK might not always meet those requirements.
Compound fertilizers
Compound fertilizers, also known as mixed fertilizers, differ from complex fertilizers primarily in their method of preparation.
(i) single nutrient or two-nutrient intermediates granulated together
(ii) Using straight fertilizers or intermediates mixed together to form a blend, each granule maintaining its original composition
(iii) A mixture of powders
Compound fertilizers perform essentially the same as their components.
The physical characteristics, storage, handling, and application characteristics of granular compound fertilizers are influenced by the manufacturing process. Nevertheless, compound fertilizers are generally safe to use as long as the coating, packaging, and storage conditions are good.
It’s also critical that the components of granular mixtures are homogenous in size and shape to avoid segregation.
Compared to granulated fertilizers, powdered fertilizers have poor storage properties and are difficult to apply uniformly. Distributors are limited in their ability to apply them.
Examples of Compound fertilizers
Granular Compound Fertilizers
Compound fertilizers are usually produced in factories using straight nitrate, phosphorus and potassium fertilizers, sometimes using two-nutrient intermediate fertilizers such as MAP.
The intermediates are usually in powder form or are slurries that are fed into a granulating plant, typically a large rotating drum.
Water or steam is added as needed, and rotation causes the formation of granules which are dried, screened for size, and bagged or bulk stored. The composition of granular compound fertilizers depends mainly on their agronomic suitability and availability. Using urea and superphosphate together can cause the phosphorus to lose water solubility and hence it is not preferred to mix such substances to make compound fertilizers.
Powdered Mixed Fertilizers
Multinutrient fertilizers are made by mixing powdered (or crystalline) straight fertilizers together on the farm, thereby reducing the number of fertilizer applications needed per field.
It is possible to formulate powder mixtures with a wide range of nutrient ratios by combining and adjusting ingredients. For example, an 8-8-8 fertilizer can be prepared by mixing Ammonium sulphate, 20.6% N + SSP, 16.5% P205 + Potassium chloride, 60% K2O ( 39% + 48% + 14% = 100%).
Compared to granular compound fertilizers, powder compound fertilizers are more affordable. However, it has some disadvantages such as: it has short term storage capabilities, the application is more time consuming and less uniform and some of the more concentrated intermediates such as ammonium nitrate and urea cannot easily be used.
Bulk Blends
By mixing or blending granular intermediates such as CAN, MAP, and potassium chloride, the cost of re-granulation can be avoided. Bulk blending involves blending granular intermediates with compatible properties. The compatible properties such as granule size, surface properties, and density should match so that there is no segregation during storage, handling and application.
Bulk blending eliminates bagging costs, and since bulk-blended fertilizer is prepared and sold immediately before application, storage factors are no longer relevant.
The bulk blending of fertilizer is primarily developed in the United States. It is typically applied by the suppliers on contract basis, thus, the farmer’s operations are simplified as large capacity equipment belonging to the contractor can be used for application.
Fluid Mixed Fertilizers
There are two types of liquid mixed fertilizers: clear liquids and suspension fertilizers.
Clear liquids are solutions in water that contain primary nutrients and are designed to not precipitate or salt out at prevailing temperatures since such deposits are hard to remove.
Ammonium nitrate, urea, ammonium phosphate or phosphoric acid, and potassium chloride are the most common nutrient sources. Concentrations achievable are considerably lower than with solid fertilizers, for example about 9-9-9 compared with 17-17-17.
Suspension fertilizers contain a small quantity of special clay, which delays the settling from the suspension of any salts that crystallize out. Thus, it is possible to achieve a higher level of concentration than clear liquids, but not as high as solids, and even high-quality ingredients are not required. However, suspension fertilizers require continuous agitation in storage and specialized application equipment.
Over solid fertilizers, fluid mix fertilizers have several advantages, namely reduced labor requirements, contract application options, and the ability to combine herbicides with fertilizers.
Secondary major nutrient fertilizers
The secondary major nutrients are calcium (Ca), sulphur(S) and magnesium(Mg). Although the uptake of calcium, sulphur, and magnesium by plants is quite substantial, it is rarely as large as those of nitrogen, phosphorus, and potassium.
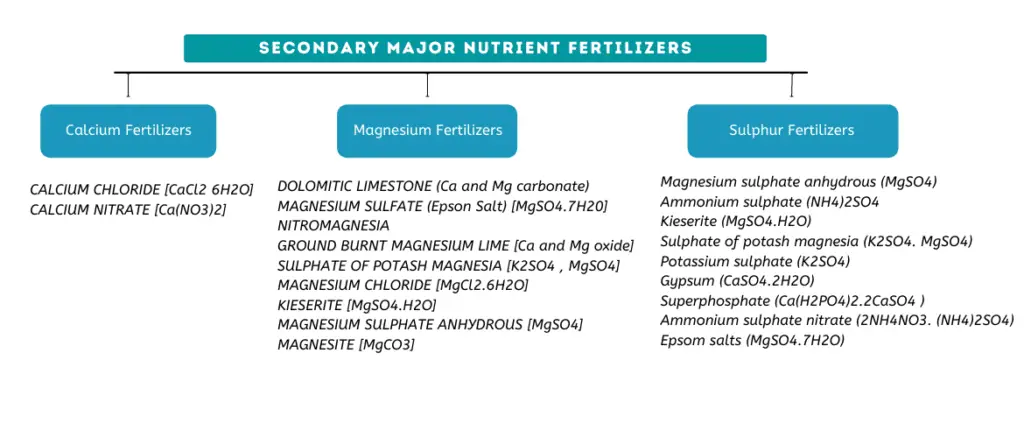
Calcium Fertilizers
In soils, plants, and liming materials, calcium content may be expressed as calcium oxide (CaO) or as elemental calcium, with a factor of 0.72 between the two.
Total calcium content in soils varies greatly depending on the parent material and can be substantial in soils formed from limestones, igneous rocks such as granites, syenites, diorites, gneisses and schists.
In contrast to this, soils derived from sandstones and shales that are noncalcareous in humid areas may contain little calcium.
A liberal application of sodium nitrate over time or repeated applications of irrigation water with a high sodium chloride content may produce an alkaline soil in which sodium is the dominant cation instead of calcium.
Regardless of the total amount of calcium in the soil, the calcium present in the soil’s base exchange complex provides readily available calcium to plants. The lower the pH value (i.e. the higher the acidity) and the lower the exchange capacity value, the less calcium is exchangeable. Calcium deficiency is particularly harmful to fruits and vegetables.
CALCIUM CHLORIDE [CaCl2 6H2O]
It is almost completely water soluble and contains 15% calcium. Because of its highly water soluble nature, it is a good candidate for foliar nutrient application.
CALCIUM NITRATE [Ca(NO3)2]
Calcium nitrate, also knon as Norgessalpeter, is also a highly water soluble calcium ferltilizer. It contains 26.5% calcium in the form of calcium oxide and 15.5% Nitrogen.
Magnesium Fertilizers
The magnesium content in soil, plants, or materials containing magnesium is usually expressed either as magnesium oxide (MgO) or as elemental magnesium, with a conversion factor of 0.61.
The soil magnesium content ranges from a trace to as much as 1 per cent. Magnesium is well supplied to arid areas or soils with high clay content, while sandy soils in high rainfall areas tend to have a low magnesium content because leaching removes it. Excessive potassium application can worsen magnesium deficiency. The soil exchange complex normally provides magnesium to the crop.
As compared with potassium and calcium, magnesium uptake by crops is much lower. Up until the last two decades, magnesium deficiency was rare, but now it is readily apparent in many crops, particularly potatoes, sugarbeets, brassicas, and maize.
It is best to correct magnesium deficiencies before plant establishment, using a variety of soil application treatments such as dolomitic limestone, kieserite, and various potassium magnesium fertilizers. Magnesium-containing NPK fertilizers are also available.
Considering economic factors and whether liming is needed determines the choice of magnesium fertilizer. Magnesium deficiency being observed during crop growth may be alleviated with foliar sprays of magnesium sulfate (Epsom salts).
DOLOMITIC LIMESTONE (Ca and Mg carbonate)
This magnesium fertilizer contains 5-20% magnesium in the form of MgO (magnesium oxide). It also contains 20-45% calcium oxide (CaO)
MAGNESIUM SULFATE (Epson Salt) [MgSO4.7H20]
This contains 16% MgO and 13% Sulphur
NITROMAGNESIA
This contains 7% MgO and 20% Nitrogen and 15% Sulphur
GROUND BURNT MAGNESIUM LIME [Ca and Mg oxide]
This contains 9-33% MgO and 26-58% calcium oxide (CaO)
SULPHATE OF POTASH MAGNESIA [K2SO4 , MgSO4]
This contains 10-18% MgO, 22-30% potassium oxide (K2O) and 16-22% sulphur(S)
MAGNESIUM CHLORIDE [MgCl2.6H2O]
This contains 20% MgO
KIESERITE [MgSO4.H2O]
This contains 27% MgO and 22% sulphur
MAGNESIUM SULPHATE ANHYDROUS [MgSO4]
This contains 33% MgO and 26.5% sulphur
MAGNESITE [MgCO3]
This contains 45% MgO
Sulphur Fertilizers
Sulphur is a highly mobilized element in soils. When soil biomass breaks down, it is mineralized into the sulfate form that crops can absorb. It is very easy for sulphate to leach from soil. Sulfur is dissolved in rainfall and deposited in soil by dry deposition but amounts vary depending on rainfall and fossil-fuel burning.
Precipitation amounts range from a few kilograms per hectare per year to over 100 kilograms. Sulphur deficiency may occur at the lower end of this spectrum.
Among brassica crops and legumes, sulphur uptake can reach 40-60 kg/ha. There is a prevalence of sulphur deficiency among these crops on every continent.
The following methods can be used to correct sulphur deficiency:
- Using sulphur-containing fertilizers like ammonium sulfate or superphosphate
- Gypsum, which is a calcium source and an ameliorant that corrects alkalinity
- By applying elemental sulphur, though this should only be used on very alkaline soils because of its soil acidifying effect; in some soils oxidation of the applied sulphur may be slow.
- Magnesium sulphate anhydrous (MgSO4), Ammonium sulphate (NH4)2SO4, Kieserite (MgSO4.H2O), Sulphate of potash magnesia (K2SO4. MgSO4), Potassium sulphate (K2SO4), Gypsum (CaSO4.2H2O), Superphosphate (Ca(H2PO4)2.2CaSO4 ), Ammonium sulphate nitrate (2NH4NO3. (NH4)2SO4), Epsom salts (MgSO4.7H2O) can be used as sulphur suplimentation fertilizers depending on the needs and circumstances.
Micronutrient Fertilizers
Micronutrients, such as iron, zinc, copper, manganese, boron, molybdenum, and chlorine, are used by plants in very small amounts, usually in terms of grams per hectare. However, even a few grams can mean the difference between high yields and crop failure.
Some elements are beneficial but not essential for crop growth, including cobalt, selenium, vanadium, nickel, lithium, silicon, and aluminum. These elements are not mentioned here.
Plants with micronutrient deficiencies display characteristic symptoms, but corrective measures may be too late once the symptoms appear, since the damage has already been done. When micronutrients are applied at this stage, they may not fully compensate for earlier deficiencies, resulting in a lower yield.
In order to ensure proper growth and development of the crop, it is necessary to determine whether the soil which the crop will be grown on contains sufficient micronutrients or if it is deficient in one or more micronutrients, and then to take corrective measures accordingly. Micronutrients should not be recommended as a blanket treatment in all soils and cropping situations; such an approach might actually cause more harm than good because of toxicity.
The amount or level of nutrients required for optimum growth of the plant is called the critical level. Different soils, different species, and even different varieties will have different critical levels of nutrition requirement.
Micronutrient forms and their rate of application
| Micronutrient | Form and amount required / Ha | Spray Application Proportion |
|---|---|---|
| Iron | Ferrous sulphate (FeSO4 7H2O), 10 kg/ha | 0.4 percent ferrous sulphate + 0.2 percent lime |
| Zinc | Zinc sulphate (ZnSO4 7H2O) , zinc oxide (ZnO) , 10-50 kg/ha | 0.5 percent zinc sulphate + 0.25 percent lime |
| Manganese | Manganese sulphate ( MnSO4 7H2O) , 10-50 kg/ha | 0.6 percent manganese sulphate + 0.25 percent lime |
| Copper | Copper sulphate (CuSO4), 10-50 kg/ha | 0.1 percent copper sulphate + 0.05 percent lime |
| Boron | Borax (Na2B4O 10H2O), 5-20 kg/ha | 0.2 percent borax |
| Molybdenum | Sodium molybdate (Na2MoO42H2O) , 0.1-0.5 kg/ha | 0.1-0.2 percent solution of ammonium molybdate |